Timeline CPPopt
20 years of CPPopt
More than twenty years of research has been performed on the cerebral hemodynamic parameter ‘optimal
cerebral perfusion pressure’ or CPPopt. The rationale behind these studies are to provide a practical clinical target (or range) that incorporates autoregulation information (‘feedback’) to fine-tune cerebral well being after a major neurological event. Over the years there is a growing evidence that each individual patient has his or her own dynamic CPPopt or CPP autoregulation ‘safe’ range. Most of the research has been performed in traumatic brain injury (TBI) patients admitted to the intensive care and therefore ‘optimal’ cerebral perfusion strategies are often linked to ICP, PRx and CPP. However, other cerebral can be used instead of ICP and more or less the same methodological principles apply to this. A short overview of the important parameters or key mechanisms important for understanding autoregulation guided therapy or CPPopt is provided below.
Intracranial pressure (ICP)
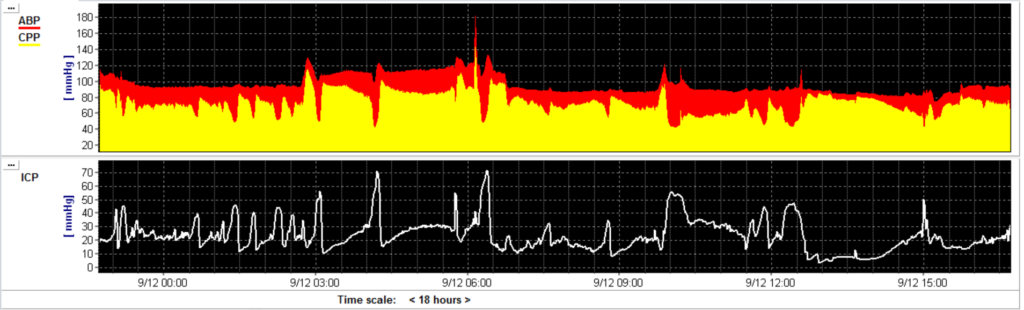
The pressure in the brain should be well regulated because of the limited volume in the skull. Particularly, in patients with severe TBI the intracranial pressure (ICP) is increased because of space occupying lesions, edema, hyperremia and/or a increased cerebral perfusion pressure with impaired cerebral autoregulation. From the famous pressure-volume curve characteristics, the measured ICP is an accurate reflexion of the swelling (volume) of the brain. Current guidelines recommend to keep ICP <22 mmHg. TBI management protocols are focused on controlling ICP. However, more research demonstrates that just controlling one cerebral parameter is probably not gaining enough benefit for a heterogenic TBI population.
Cerebral perfusion pressure (CPP)
Cerebral perfusion pressure (CPP) is calculated as arterial blood pressure minus ICP. The CPP is the surrogate parameter to represent adequate CBF during intensive care stay. Current guidelines recommend to keep CPP between 60-70 mmHg but these fixed threshold have changed over the years.
Cerebral autoregulation (CA)
It is hypothesized that there is an important cerebral mechanism that keeps the cerebral blood flow (CBF) constant by arteriolar vasodilatation and vasoconstriction. This autoregulation mechanism was visualised by the Larsen’s autoregulation plateau which indicated that over a large CPP range CBF remains constant (50-150 mmHg). An increase in CPP causes vasoconstriction whereas a decrease in CPP causes vasodilatation in the cerebral arterioles. The range in which this mechanism is valid is the range between the lower limit of cerebral autoregulation and the upper limit of cerebral autoregulation. Below the lower limit of cerebral autoregulation the brain is more at risk for ischaemia whereas a CPP value above the upper limit hyperremia might cause cerebral damage.
Pressure reactivity index (PRx)
PRx is the moving Pearson correlation between the slow waves in ICP and ABP. In 1997 the pressure reactivity index (PRx) was firstly described as acting dynamically to a decrease in CPP. This indicated that PRx could be used as an index for the cerebrovascular reactivity [1]. Cerebrovascular pressure reactivity (PRx) is distinct from autoregulation, but it cannot be denied that it is strongly related to it [2]. It has been shown in animal models that PRx accurately delineates the lower limits of autoregulation [3]. Numerous (retrospective) observations in different patient populations led to important correlations between averaged PRx and outcome [2].
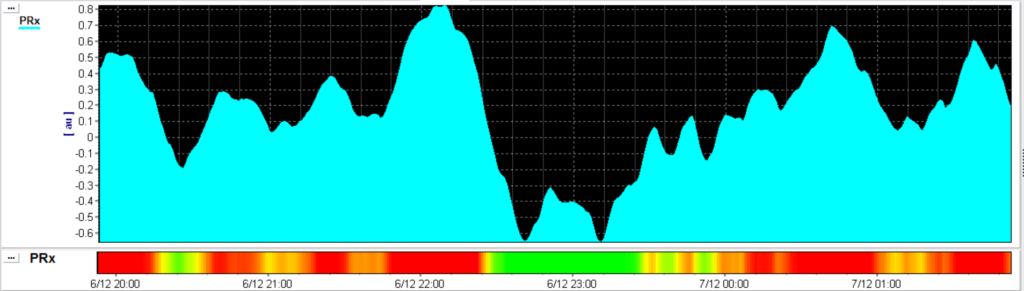
However, a fundamental requirement for conducting research on cerebral autoregulation (CA), and consequently for calculating the Pressure Reactivity Index (PRx), is the presence of a sufficient number of slow ABP-waves. To induce slow ABP waves, Tas et al. adopted a method previously employed in piglet studies, involving the manipulation of ventilator pressure through the sigh-function. Their research findings revealed an enhanced level of reliability in PRx measurements (reduced variability), all without other clinically relevant changes in TBI patients with ICP<22 mmHg [4].
Optimal cerebral perfusion pressure (CPPopt)
In 2002 Steiner et al. plotted the CPP against the PRx index [5]. He showed retrospectively that long time monitoring (24 hrs) detects an ‘optimal’ CPP, meaning that there is a CPP value in which the PRx is the lowest and so the cerebral autoregulation might work ‘optimal’. Although this was a great insight, measuring 24 hours before generating an output is not clinical applicable on the intensive care unit.
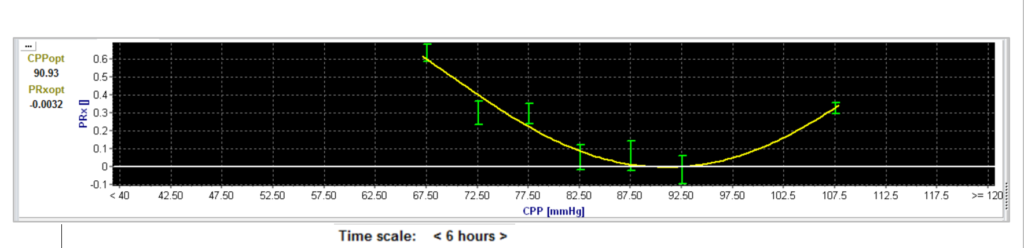
For making CPPopt clinically interesting, Aries et al. managed to use a 4-hr moving time window to get CPPopt automatically and during 55% of the monitoring time [6]. The authors showed retrospectively that deviation of calculated CPPopt was related to patient outcome. In addition, Liu et al. managed to apply a multiwindow averaging approach increased the percentage of time that CPPopt was continuously available from 51% towards 94% [7,8].
To enhance the reliability and stability of CPPopt further, Beqiri et al. fine-tuned the CPPopt calculation, resulting in significantly improved stability, acceptable yield, and a comparable outcome prediction [9]. With these improvements, the calculation became suitable for prospective use in the CPPOpt Guided Therapy: Assessment of Target Effectiveness (COGiTATE) study.
In the initial feasibility and safety study, COGiTATE compared two groups: one targeting the standard CPP range [60-70] and the other targeting a CPP value based on CPPopt. The results demonstrated a significant increase in the percentage of time that CPP was close to the CPPopt trendline (46%), as compared to the previously computed historical data (36%). Also, there were no differences in the therapeutic intensity level scores and other safety endpoints, concluding that targeting an individual and dynamic CA-guided CPP is feasible and safe in TBI patients [10, 11].
More information on PRx and CPPopt
Donnelly, J., Aries, M. J., Czosnycka, M. (2015). Further understanding of cerebral autoregulation at the bedside: possible implications for future therapy. Expert Review of Neurotherapeutics, 15(2), 169–85. http://doi.org/10.1586/14737175.2015.996552
Downloads
Presentation MJH. Aries: 15 years of CPPopt (Cambridge, February 2017)
Presentations CPPopt Satellite Symposium (Boston, June 2016)
References
[1] Czosnyka M, Smielewski P, Kirkpatrick P, et al. Continuous Assessment of the Cerebral Vasomotor Reactivity in Head Injury. July 1997. Neurosurgery, 4: 11-19.
[2] Czosnyka M, Brady K, Reinhard M, Smielewski P, Steiner LA. Monitoring of cerebrovascular autoregulation: facts, myths, and missing links. Neurocrit Care 2009; 10:373-386.
[3] Brady KM, Lee JK, Kibler KK, Easley RB, Koehler RC, Shaffner DH. Continuous measurement of autoregulation by spontaneous fluctuations in cerebral perfusion pressure: comparison of 3 methods. Stroke 2008; 39:2531-2537.
[4] Tas, J., Bos, K. D. J., Le Feber, J., Beqiri, E., Czosnyka, M., Haeren, R., van der Horst, I. C. C., van Kuijk, S. M. J., Strauch, U., Brady, K. M., Smielewski, P., & Aries, M. J. H. (2022). Inducing oscillations in positive end-expiratory pressure improves assessment of cerebrovascular pressure reactivity in patients with traumatic brain injury. Journal of applied physiology (Bethesda, Md. : 1985), 133(3), 585–592. https://doi.org/10.1152/japplphysiol.00199.2022
[5] Steiner, L. A., Czosnyka, M., Piechnik, S. K., Smielewski, P., Chatfield, D., Menon, D. K., & Pickard, J. D. (2002). Continuous monitoring of cerebrovascular pressure reactivity allows determination of optimal cerebral perfusion pressure in patients with traumatic brain injury. Critical care medicine, 30(4), 733–738. https://doi.org/10.1097/00003246-200204000-00002 [Add to Citavi project by DOI]
[6] Aries, M. J., Czosnyka, M., Budohoski, K. P., Steiner, L. A., Lavinio, A., Kolias, A. G., Hutchinson, P. J., Brady, K. M., Menon, D. K., Pickard, J. D., & Smielewski, P. (2012). Continuous determination of optimal cerebral perfusion pressure in traumatic brain injury. Critical care medicine, 40(8), 2456–2463. https://doi.org/10.1097/CCM.0b013e3182514eb6
[7] Liu, X., Maurits, N. M., Aries, M. J. H., Czosnyka, M., Ercole, A., Donnelly, J., Cardim, D., Kim, D. J., Dias, C., Cabeleira, M., & Smielewski, P. (2017). Monitoring of Optimal Cerebral Perfusion Pressure in Traumatic Brain Injured Patients Using a Multi-Window Weighting Algorithm. Journal of neurotrauma, 34(22), 3081–3088. https://doi.org/10.1089/neu.2017.5003
[8] Güiza, F, Meyfroidt, G, Lo, T-YM, Jones, PA, et al. Continuous optimal CPP based on minute-by-minute monitoring data: A study of a pediatric population (2016) Acta Neurochirurgica, Supplementum, 122, pp. 187-191.
[9] Beqiri, E., Ercole, A., Aries, et al. Towards autoregulation-oriented management after traumatic brain injury: increasing the reliability and stability of the CPPopt algorithm. Journal of clinical monitoring and computing, 2023.
[10] Beqiri, E., Smielewski, P., Robba, C., Czosnyka, M., Cabeleira, M. T., Tas, J., Donnelly, J., Outtrim, J. G., Hutchinson, P., Menon, D., Meyfroidt, G., Depreitere, B., Aries, M. J., & Ercole, A. (2019). Feasibility of individualised severe traumatic brain injury management using an automated assessment of optimal cerebral perfusion pressure: the COGiTATE phase II study protocol. BMJ open, 9(9), e030727. https://doi.org/10.1136/bmjopen-2019-030727
[11] Tas, J., Beqiri, E., van Kaam, R. C., Czosnyka, M., Donnelly, J., Haeren, R. H., van der Horst, I. C. C., Hutchinson, P. J., van Kuijk, S. M. J., Liberti, A. L., Menon, D. K., Hoedemaekers, C. W. E., Depreitere, B., Smielewski, P., Meyfroidt, G., Ercole, A., & Aries, M. J. H. (2021). Targeting Autoregulation-Guided Cerebral Perfusion Pressure after Traumatic Brain Injury (COGiTATE): A Feasibility Randomized Controlled Clinical Trial. Journal of neurotrauma, 38(20), 2790–2800. https://doi.org/10.1089/neu.2021.0197 [Add to Citavi project by DOI]
